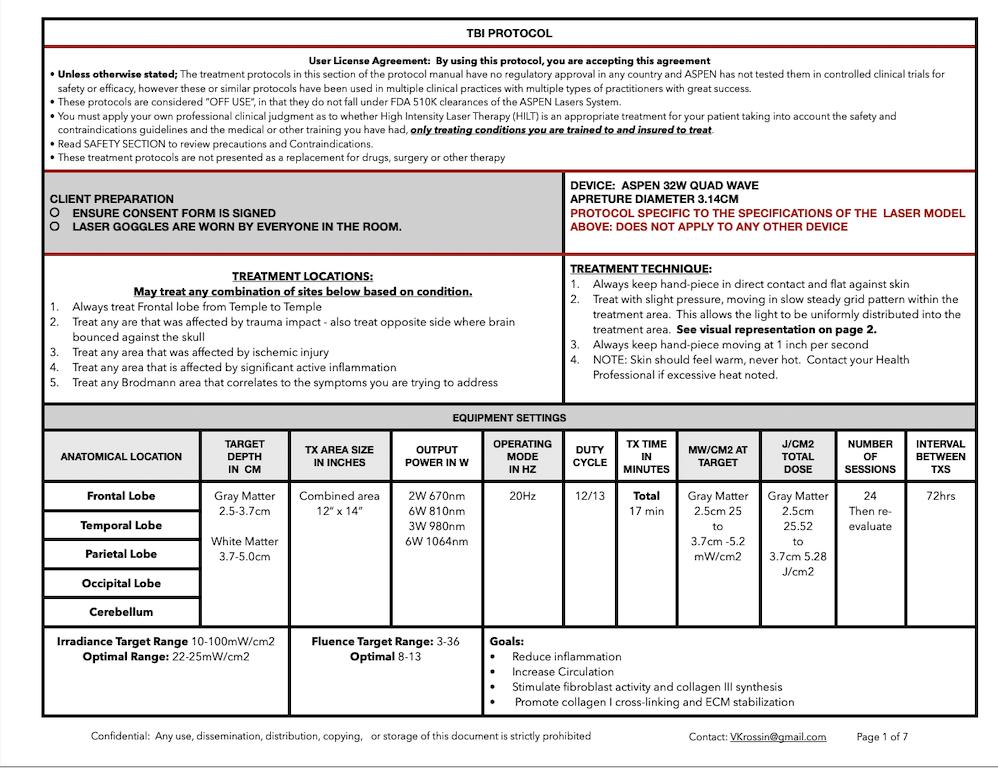
IN THIS LESSON
Standard Dosing Parameters
Clinical protocols for transcranial brain photobiomodulation typically employ wavelengths between 810-1070 nm with power densities (irradiance) ranging from 50-400 mW/cm². Energy density (fluence) delivered per session ranges from 13-185 J/cm², with treatment durations of 6-24 minutes per session. Most successful protocols utilize pulsed wave delivery rather than continuous wave, with pulse frequencies of either 10 Hz (alpha band) or 40 Hz (gamma band).
The 10 Hz pulsing frequency aligns with brain alpha oscillations and has demonstrated effectiveness in promoting skin wound healing, reducing inflammation and brain damage in TBI, enhancing mitochondrial function and neurogenesis, and mitigating amyloid-beta accumulation and cognitive impairment in AD models. The 40 Hz gamma frequency has shown particular promise for activating microglia to reduce amyloid burden, improving cognitive function with more pronounced changes in brain electrical activity, and enhancing glymphatic clearance through multisensory stimulation.
Treatment frequency typically ranges from 3-7 sessions per week, with total treatment durations of 8-12 weeks for optimal results. Many protocols combine transcranial placement targeting frontal, parietal, and temporal regions with intranasal delivery to reach deeper brain structures including the ventromedial prefrontal cortex, orbitofrontal cortex, and hippocampus.
Low Power vs Class IV
Recent findings challenge current practices using low-power LED devices and suggest a need to revise treatment protocols to ensure adequate power delivery for therapeutic effects. The demonstrated safety and efficacy of high-powered PBM, particularly in treating complex neurological conditions, positions it as a promising non-invasive therapeutic option.
Future development of this technology should focus on optimizing delivery systems while maintaining necessary power levels for effective tissue penetration. Additional controlled clinical trials are warranted to further validate these findings and establish standardized treatment protocols. The potential of PBM as both a primary and adjunctive therapy merits continued investigation, particularly given its favorable risk-benefit profile and the growing need for effective treatments for neurological conditions.
The evidence suggests that when properly implemented with adequate power and appropriate parameters, PBM represents a valuable therapeutic tool that could significantly impact the treatment of various neurological conditions, offering new hope for patients with limited treatment options.
Parameter Published Optimal Range/Value
Irradiance (mW/cm²) 22–285 (most studies for cognitive effects)
Fluence (J/cm²) 15–60 common: 20–30 for neuro, 12–84 for psych, max 60)
Max Dose/Session ≤60 J/cm² (per site/session, with most studies using 15–30)
Sessions/Week 3 (e.g., Mon/Wed/Fri)
Total Sessions 18–24 (over 6–8 weeks); some protocols up to 40–72
-
Citations:
Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015;11:2191-2208.
Morries LD, Cassano P, Henderson TA. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr Dis Treat. 2015;11:2159-75.
Henderson TA, Morries LD. Multi-watt near-infrared phototherapy for the treatment of comorbid depression: An open-label single-arm study. Front Psychiatry. 2017;8:187.
Shen Q, Guo H, Yan Y. Photobiomodulation for neurodegenerative diseases: A scoping review. Int J Mol Sci. 2024;25:1625.
Moro C, Valverde A, Dole M, et al. The effect of photobiomodulation on the brain during wakefulness and sleep. Front Neurosci. 2022;16:942536.
Hamblin MR. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016;6:113-124.
Lapchak PA, Boitano PD, Butte PV, et al. Transcranial near-infrared laser transmission (NILT) profiles: Systematic comparison in four common research species. PLoS ONE. 2015;10:e0127580.
Cassano P, Petrie SR, Hamblin MR, et al. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics. 2016;3:031404.
Tedford CE, DeLapp S, Jacques S, Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med. 2015;47:312-22.
Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13-23.
Salehpour F, Rasta SH, Mohaddes G, et al. Therapeutic effects of 10-Hz pulsed wave lasers in rat depression model: A comparison between near-infrared and red wavelengths. Lasers Surg Med. 2016;48:695-705.
Naeser MA, Zafonte R, Krengel MH, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma. 2014;31:1008-17.
Xuan W, Agrawal T, Huang L, et al. Low-level laser therapy for traumatic brain injury in mice increases brain-derived neurotrophic factor (BDNF) and synaptogenesis. J Biophotonics. 2015;8:502-11.
Johnstone DM, Moro C, Stone J, et al. Turning on lights to stop neurodegeneration: The potential of near-infrared light therapy in Alzheimer's and Parkinson's disease. Front Neurosci. 2016;9:500.
Wang X, Tian F, Reddy DD, et al. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study. J Cereb Blood Flow Metab. 2017;37:3789-3802.