IN THIS LESSON
Primary Photoacceptor: Cytochrome C Oxidase
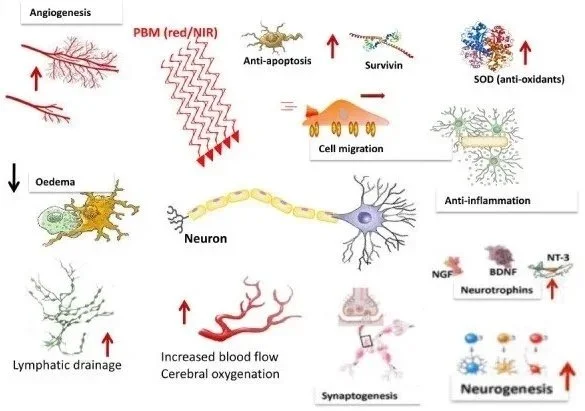
The biological effects of photobiomodulation are mediated primarily through cytochrome c oxidase (CCO), the terminal enzyme in the mitochondrial electron transport chain. CCO contains multiple chromophores with absorption peaks across the red and near-infrared spectrum, including heme a at 605 nm, CuA reduced at 620 nm, heme a3/CuB at 655 nm, and additional peaks extending into the NIR region with CuB reduced at 760 nm and CuA oxidized at 825 nm. The 810 nm wavelength demonstrates optimal absorption by CCO due to its peak alignment with the enzyme's spectral properties, making it the most widely studied and clinically validated wavelength for brain applications.
When photons are absorbed by CCO, several immediate biochemical events occur. First, nitric oxide (NO), which is normally bound to the heme iron and copper centers of CCO and competitively inhibits oxygen binding, undergoes photodissociation. This releases NO from the enzyme, allowing oxygen to rebind and restoring normal mitochondrial respiration. The freed NO molecules then diffuse to adjacent vascular smooth muscle cells where they activate soluble guanylate cyclase, increasing cyclic GMP levels and promoting vasodilation and increased cerebral blood flow.
Bioenergetic Enhancement and Cellular Signaling
The photostimulation of CCO leads to enhanced oxidative phosphorylation and increased adenosine triphosphate (ATP) production. This boost in cellular energy availability activates multiple downstream signaling cascades. The increased ATP production supports energy-dependent cellular processes including protein synthesis, ion transport, and neurotransmitter production. Additionally, PBM induces a mild, transient increase in reactive oxygen species (ROS) that serves as a secondary messenger to activate protective cellular pathways.
These signaling effects include activation of nuclear factor kappa B (NF-κB), which regulates the expression of over 100 genes involved in antioxidant, anti-apoptotic, pro-proliferative, and pro-migratory functions. The upregulation of brain-derived neurotrophic factor (BDNF) occurs through activation of the BDNF/TrkB signaling pathway, promoting neuronal survival, synaptic plasticity, and neurogenesis. Studies have also demonstrated that PBM activates PKC signaling-dependent upregulation of anti-apoptotic proteins like Bcl-2 while inhibiting pro-apoptotic proteins such as Bax.
Vascular and Anti-inflammatory Effects
Beyond direct mitochondrial effects, photobiomodulation produces significant vascular benefits. The photodissociated NO acts as a potent vasodilator, increasing regional cerebral blood flow by approximately 30% in experimental models. Clinical studies using single-photon emission computed tomography (SPECT) have demonstrated that pulsed PBM effectively enhanced regional cerebral blood flow in 66.7% of participants with traumatic brain injury. This enhanced perfusion delivers increased oxygen and glucose to metabolically compromised brain regions.
The anti-inflammatory effects of PBM are mediated through multiple mechanisms including modulation of microglial activation, reduction of pro-inflammatory cytokines, and promotion of an M2 anti-inflammatory microglial phenotype. In Alzheimer's disease models, PBM has been shown to reduce microglial proliferation and downregulate inflammatory markers while enhancing the phagocytic clearance of amyloid-beta plaques.
-
Citations:
Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015;11:2191-2208.
Morries LD, Cassano P, Henderson TA. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr Dis Treat. 2015;11:2159-75.
Henderson TA, Morries LD. Multi-watt near-infrared phototherapy for the treatment of comorbid depression: An open-label single-arm study. Front Psychiatry. 2017;8:187.
Shen Q, Guo H, Yan Y. Photobiomodulation for neurodegenerative diseases: A scoping review. Int J Mol Sci. 2024;25:1625.
Moro C, Valverde A, Dole M, et al. The effect of photobiomodulation on the brain during wakefulness and sleep. Front Neurosci. 2022;16:942536.
Hamblin MR. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016;6:113-124.
Lapchak PA, Boitano PD, Butte PV, et al. Transcranial near-infrared laser transmission (NILT) profiles: Systematic comparison in four common research species. PLoS ONE. 2015;10:e0127580.
Cassano P, Petrie SR, Hamblin MR, et al. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics. 2016;3:031404.
Tedford CE, DeLapp S, Jacques S, Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med. 2015;47:312-22.
Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13-23.
Salehpour F, Rasta SH, Mohaddes G, et al. Therapeutic effects of 10-Hz pulsed wave lasers in rat depression model: A comparison between near-infrared and red wavelengths. Lasers Surg Med. 2016;48:695-705.
Naeser MA, Zafonte R, Krengel MH, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma. 2014;31:1008-17.
Xuan W, Agrawal T, Huang L, et al. Low-level laser therapy for traumatic brain injury in mice increases brain-derived neurotrophic factor (BDNF) and synaptogenesis. J Biophotonics. 2015;8:502-11.
Johnstone DM, Moro C, Stone J, et al. Turning on lights to stop neurodegeneration: The potential of near-infrared light therapy in Alzheimer's and Parkinson's disease. Front Neurosci. 2016;9:500.
Wang X, Tian F, Reddy DD, et al. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study. J Cereb Blood Flow Metab. 2017;37:3789-3802.