IN THIS LESSON
Default Mode Network Modulation
One of the most significant discoveries in photobiomodulation research is its ability to modulate functional connectivity in the default mode network (DMN), a set of brain regions including the medial prefrontal cortex, posterior cingulate cortex/precuneus, and angular gyrus that shows synchronous activity during rest and is critically involved in self-referential processing and memory consolidation. Dysregulation of the DMN is a hallmark of Alzheimer's disease, making it an important therapeutic target.
Functional MRI studies have demonstrated that 12 weeks of PBM treatment with the Vielight Neuro device produces increased functional connectivity in the default mode network, particularly in parietal regions, representing the first report of such improvements following a non-pharmacological intervention. PBM appears to correct the imbalance of functional connectivity in damaged or diseased states, restoring connectivity between cortical areas to more normal levels. In healthy subjects, PBM modulates the strength of resting connectivity and alters task-based activation patterns.
Graph theory analysis using functional near-infrared spectroscopy has revealed that transcranial PBM causes significant changes in network global efficacy in both alpha and gamma frequency bands. The therapy increases clustering coefficient, characteristic path length, and local efficiency measures across oscillation frequency bands, indicating enhanced integration and segregation properties of brain networks. These topological changes in brain network organization correlate with the cognitive improvements observed clinically.
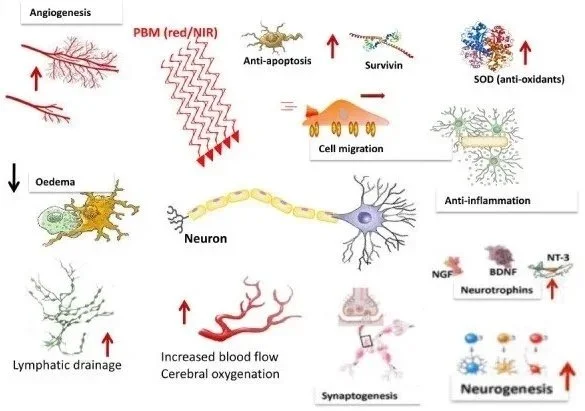
Neuroplasticity and Neurogenesis
Photobiomodulation exerts profound effects on neuroplasticity and adult neurogenesis, processes essential for learning, memory, and recovery from brain injury. PBM treatment significantly expands the pool of neural progenitor cells and newly generated immature neurons in cortical regions following ischemic stroke. The therapy reduces cell death among both neural progenitor cells and newly formed neurons while promoting the generation of mature neurons in peri-infarct zones.
The neuroplasticity effects are mediated in part through upregulation of brain-derived neurotrophic factor (BDNF) and activation of the BDNF/TrkB signaling pathway. BDNF is a critical growth factor supporting neuronal survival, differentiation, and synaptic plasticity. PBM also modulates synaptic plasticity—the ability of synapses to strengthen or weaken over time—which is fundamental to cognitive function and adaptive brain responses.
In Alzheimer's disease models, PBM not only reduces the accumulation of pathological proteins but actively promotes neuroprotection and repair. Four weeks of 670 nm transcranial PBM reduced hyperphosphorylated tau protein and neurofibrillary tangles in tau transgenic mice while simultaneously reducing amyloid-beta plaque burden in APP/PS1 Alzheimer's model mice. The treatment restored mitochondrial function, reduced oxidative stress markers, and decreased expression of inflammatory mediators.
Amyloid and Tau Protein Modulation
Recent research has demonstrated that photobiomodulation can directly impact the pathological hallmarks of Alzheimer's disease. PBM at 808 nm and 1070 nm wavelengths with 10 Hz pulsing has been shown to reduce the accumulation of amyloid-beta plaques through multiple mechanisms. The therapy activates microglia and promotes their polarization toward an M2 anti-inflammatory phenotype, which enhances phagocytic clearance of amyloid deposits. Specifically, 40 Hz light stimulation activates microglia to significantly reduce amyloid burden, an effect that can be amplified when combined with 40 Hz auditory stimulation for multisensory gamma entrainment.
Treatment with 808 nm laser at 25 mW/cm² for 2 minutes daily over five consecutive days decreased oxidative damage and suppressed both tau tangles and beta-amyloid plaques in animal models. The reduction in pathological protein aggregates correlates with improvements in learning, memory, and cognitive function, particularly when treatment is initiated during early pathological stages. Immunofluorescence and behavioral analyses confirm that laser therapy effectively ameliorates AD symptoms through these neuroprotective mechanisms.
Beyond clearing existing pathology, PBM appears to prevent further accumulation by enhancing mitochondrial function, reducing oxidative stress that promotes protein misfolding, and modulating the expression of genes involved in amyloid precursor protein processing. These multi-level effects position PBM as a disease-modifying intervention rather than merely symptomatic treatment.
-
Citations:
Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015;11:2191-2208.
Morries LD, Cassano P, Henderson TA. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr Dis Treat. 2015;11:2159-75.
Henderson TA, Morries LD. Multi-watt near-infrared phototherapy for the treatment of comorbid depression: An open-label single-arm study. Front Psychiatry. 2017;8:187.
Shen Q, Guo H, Yan Y. Photobiomodulation for neurodegenerative diseases: A scoping review. Int J Mol Sci. 2024;25:1625.
Moro C, Valverde A, Dole M, et al. The effect of photobiomodulation on the brain during wakefulness and sleep. Front Neurosci. 2022;16:942536.
Hamblin MR. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016;6:113-124.
Lapchak PA, Boitano PD, Butte PV, et al. Transcranial near-infrared laser transmission (NILT) profiles: Systematic comparison in four common research species. PLoS ONE. 2015;10:e0127580.
Cassano P, Petrie SR, Hamblin MR, et al. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics. 2016;3:031404.
Tedford CE, DeLapp S, Jacques S, Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med. 2015;47:312-22.
Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13-23.
Salehpour F, Rasta SH, Mohaddes G, et al. Therapeutic effects of 10-Hz pulsed wave lasers in rat depression model: A comparison between near-infrared and red wavelengths. Lasers Surg Med. 2016;48:695-705.
Naeser MA, Zafonte R, Krengel MH, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma. 2014;31:1008-17.
Xuan W, Agrawal T, Huang L, et al. Low-level laser therapy for traumatic brain injury in mice increases brain-derived neurotrophic factor (BDNF) and synaptogenesis. J Biophotonics. 2015;8:502-11.
Johnstone DM, Moro C, Stone J, et al. Turning on lights to stop neurodegeneration: The potential of near-infrared light therapy in Alzheimer's and Parkinson's disease. Front Neurosci. 2016;9:500.
Wang X, Tian F, Reddy DD, et al. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study. J Cereb Blood Flow Metab. 2017;37:3789-3802.